Medical Waste Management Plan
Chaffey College facilities: Rancho Cucamonga Campus, Chino Campus, and Fontana Campus.
Sam Gaddie, Chemical Hygiene Officer, is responsible for implementation of the plan.
| Date: | July 12, 2018 |
|---|---|
| Generator Facility: | Chaffey College – Rancho Cucamonga Campus |
| Site Address: | 5885 Haven Avenue |
| City: | Rancho Cucamonga |
| State: | CA |
| Zip: | 91737-3002 |
| Telephone: | (909) 652-6000 |
| Generator Facility: | Chaffey College – Chino Campus |
|---|---|
| Site Address: | 5897 College Park Avenue |
| City: | Chino |
| State: | CA |
| Zip: | 91710-8241 |
| Telephone: | (909) 652-8000 |
| Generator Facility: | Chaffey College – Fontana Campus |
|---|---|
| Site Address: | 16855 Merrill Avenue |
| City: | Fontana |
| State: | CA |
| Zip: | 92335-8626 |
| Telephone: | (909) 652-7400 |
Types of wastes generated:

| Generated | Type of waste | Description |
|---|---|---|
| x | Laboratory Waste | specimen or microbiologic cultures, stocks of infectious agents, live and attenuated vaccines, and culture mediums. |
| x | Blood or body fluids | liquid blood elements or other regulated body fluids, or articles contaminated with blood or body fluids. |
| x | Sharps | Syringes, needles, blades, broken glass. |
| Contaminated animals | animal carcasses, body parts, bedding materials. | |
| Surgical specimens | Human or animal parts or tissues removed surgically or by autopsy. | |
| Isolation waste | Waste contaminated with excretion, exudate, or secretions from humans or animals who are isolated due to highly communicable diseases. (Centers for Disease Control, Biosafety Level 4)* | |
| Wastes contaminated | with fixatives or chemotherapeutic agents. | |
| Other | (Specify): |
Estimate of monthly quantity generated: 175 pounds
*Biosafety Level 4 viruses and diseases are: Crimean-Congo hemorrhagic fever, Tick-borne
encephalitis virus complex (Absettarov, Hanzalova, Hypr, Kumlinge, Kyasanur Forest
disease, Omsk hemorrhagic fever, and Russian Spring-Summer encephalitis), Marburg
disease, Ebola, Junin virus, Lassa fever virus, Machupo virus, Lujo, Dengue, Herpes
B virus, H7N9, Nipah, Hendra, Middle-Eastern Respiratory Syndrome-Coronavirus, and
hemorrhagic fever agents or viruses as yet identified.
Method of treatment performed on site
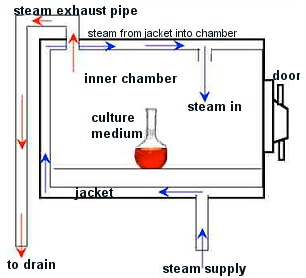
- Incineration
- Autoclave
- Microwave
- Other
Describe the following methods used for medical waste
Segregation from other wastes
Medical/Biohazard Waste is segregated from all other wastes at the point of origin and during storage. Surgical specimen waste may be disposed of as solid waste.
Containment or packaging procedures
Biohazardous waste is contained in red biohazard bags, which meet the 165 gram dropped dart impact resistant test. The biohazard bags must be in rigid, properly labeled containers for storage, transportation, and delivery. The medical/biohazard waste containers are leak resistant, have tight-fitting lids and are clean and in good repair. Sharps waste is contained in rigid, puncture-resistant, leak-resistant sharps containers.
Labeling procedures
Biohazardous wastes are labeled “BIOHAZARD” with the international biohazard symbol. Sharps waste is labeled “Sharps Waste” with the international biohazard symbol. All biohazardous waste and sharps waste containers must include information about the site/location where the waste was generated.
Collection procedures
On the Rancho Cucamonga and Chino campuses, in areas other than the Microbiology Lab, generation of medical/biohazard waste is sporadic. This waste is collected by custodial staff and transported to the microbiology biohazard accumulation site in BL-111 (Rancho Cucamonga campus) or CHHC-103 (Chino campus). All biohazardous waste must be stored, transported, and delivered in rigid, properly labeled containers. As a routine operation, medical/biohazard waste containers are checked weekly by the Dean of Mathematics and Science; the Dean of Health Sciences; the Dean of Kinesiology, Nutrition and Athletics; the Director of the Student Health Services; and the Director of the Child Development Center; or his/her designee. Within thirty days of the containers having medical/biohazard waste, Maintenance and Operations will be notified to transport the waste to the microbiology biohazard collection site in BL-111 (Rancho Cucamonga campus) or CHHC-103 (Chino campus). Biohazardous waste is then steam sterilized (autoclaved) within seven days if greater than twenty pounds or within thirty days if less than twenty pounds.
Procedures
The biohazardous medical waste generated in the microbiology labs is collected in biohazard bags and treated by autoclaving when the bag is full or within seven days, whichever comes first.
All sharps waste is to be treated as biohazardous waste. The biohazardous waste sharps are collected in sharps containers and treated by steam sterilization (autoclaving) within 30 days of the container reaching full, after which the containers are disposed of as solid waste. Collection of any medical/biohazard waste that might be generated by the Child Development Center, located on the Rancho Cucamonga campus, will follow the same containment, labelling and collection guide lines outlined above. Maintenance and Operations will be notified to transport the waste to the microbiology biohazard accumulation site in BL-111. As a routine operation, medical/biohazard waste containers are checked weekly by the Director of the Child Development Center or his/her designee.
Storage Methods (including duration and temperature controls): Biohazardous medical waste and sharps waste (after reaching capacity) are stored at room temperature for less than 7 days if greater than 20 pounds or less than 30 days if under 20 pounds. Only authorized individuals have access to the secure, storage areas. The areas are marked clearly with warning signs on the exterior door bearing the biohazard symbol and the following legend in English and Spanish “CAUTION-BIOHAZARDOUS WASTE STORAGE AREA-UNAUTHORIZED PERSONS KEEP OUT!” The signs are legible from 25 feet. The storage areas are not accessible to animals or destructive natural elements.
Disinfection procedures (containers, wastes, linen): The reusable biohazardous medical waste containers are decontaminated with a dilute bleach solution (10%) or other CAL-OSHA-approved cleaner after each use following the manufacture’s recommendations for disinfecting.
Treatment methods: Medical/biohazard waste from the microbiology program is treated via steam sterilization (autoclave) and then disposed of as solid waste. Sharps wastes is collected in sharps containers and treated by steam sterilization (autoclaving) within 30 days of the container reaching full, after which the containers are disposed of as solid waste.
All other waste from Health Sciences and from Athletics, as well as from Student Health Services and the Child Development Center is collected, transported and delivered in rigid, properly labeled containers and treated via steam sterilization (autoclave) within thirty days of collection and disposed of as solid waste.
Standard operating procedures are written and posted in BL-111 (Rancho Cucamonga campus), CHHC-103 (Chino campus), and FNAC-203 (Fontana campus) near the steam sterilizer and include the use of heat-sensitive tape on each bag. A spore indicator (Bacillus sterothermophilus) is used once per month by placing at the center of a load that is processed under standard conditions to confirm attainment of adequate sterilization conditions. The records for these tests are maintained in BL-111 (Rancho Cucamonga campus), CHHC-103 (Chino campus), and FNAC-203 (Fontana campus) for three years. A recording of each run showing attainment of 121 degrees Celsius for at least one-half hour is maintained for three years. STERIS, under the conditions of our maintenance contract, checks thermometers at least annually. Records of the calibration checks are maintained in BL-111 (Rancho Cucamonga campus), CHHC-103 (Chino campus), and FNAC-203 (Fontana campus) for three years.
In event of an Emergency (treatment system breaks down, hauler unable to pick up waste, spill, etc.): In the event of a biohazardous spill, all areas will clean and disinfect the infectious body fluid spills or biohazardous medical waste materials using a biohazardous decontamination kits approved by OSHA, CDC and NCCLS. All involved staff will receive biohazardous decontamination procedure training prior to beginning any work with exposure or potential exposure to biohazardous materials and /or waste.
The large autoclaves used at the Rancho Cucamonga and Chino campuses are under maintenance contracts with STERIS and receives regular maintenance. In the event of a breakdown, STERIS responds within two days and repairs the equipment. In the event of a total breakdown, a replacement machine will be purchased immediately and in the interim, medical waste will be picked up by Amberwick Corporation (located at 2304 W. 16th Street, Long Beach, CA 90813, 1-800-300-9990).
The small, table-top autoclave at the Fontana campus will be maintained in-house. In the event of a total breakdown, a replacement machine will be purchased immediately and in the interim, medical waste will be picked up by Amberwick Corporation (located at 2304 W. 16th Street, Long Beach, CA 90813, 1-800-300-9990).

